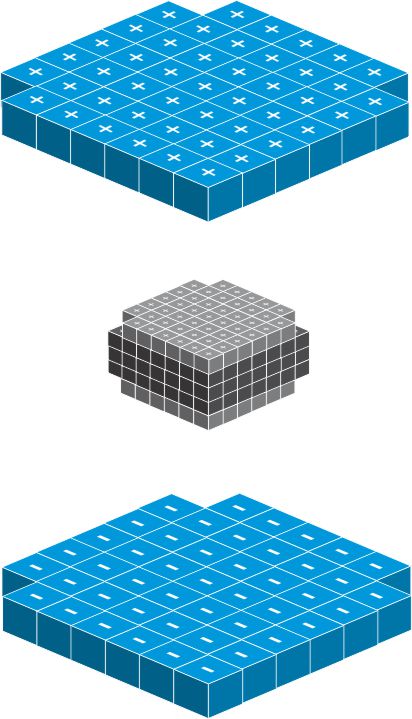
All matter including solid, liquid or gas is made up of atom. The atom is one of the most fundamental building blocks of matter. An atom is the smallest unit of an element that retains the properties of that element. It is composed of protons, neutrons, and electrons. The outer part of the atom consists of a number of electrons. The nucleus is the center of the atom: it contains the nucleons, the protons and neutrons. The protons and neutrons are bound very tightly to form the nucleus. The nucleus is very tiny, only about 1/100,000th size of atom, but it is very heavy, almost the entire mass of the atom is on the nucleus.
Proton has one unit positive electric charge
Neutrons have no electric charge.
Electron has one unit negative electric charge.
The mass of proton is about 1 atomic mass unit (amu).
The mass of neutron is slightly over 1 amu.
The mass of electron is about 1/1836th of proton.
The atom is electrically neutral since it consists of the same number of electron and proton.
Electron, proton, neutron all have half spin .
Hydrogen-1 nuclide has only one proton as its nucleus.
Beside the hydrogen-1, all other nucleus must consist of both proton and neutron.
The elements differ from each other in the number of protons.
Inside the nucleus, the protons and neutrons act like a unit cube which stack together to form the nucleus: the neutron form the kernel of the nucleus whiles the proton is located on the surface of nucleus.
Spin up proton
Spin +1/2

Spin down proton
Spin -1/2

Spin up neutron
Spin 1/2

Spin down neutron
Spin -1/2

The nuclide is characterized by the number of proton, the number of neutron, and the nuclear spin of the nucleus.
Nuclear Isotopes are nuclides with equal proton number but different neutron numbers.
Nuclear isotones are nuclides with equal neutron number but different proton numbers.
Nuclear isomers are nuclides with equal proton number and equal neutron number, but different nuclear spin.
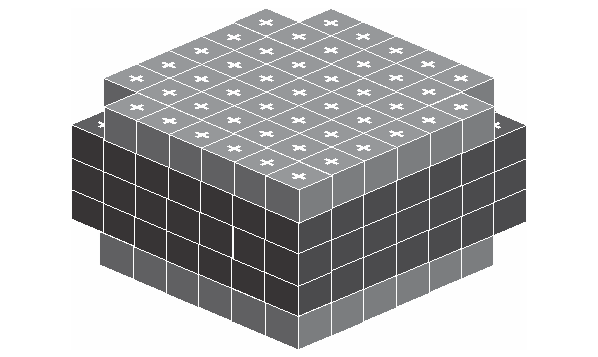
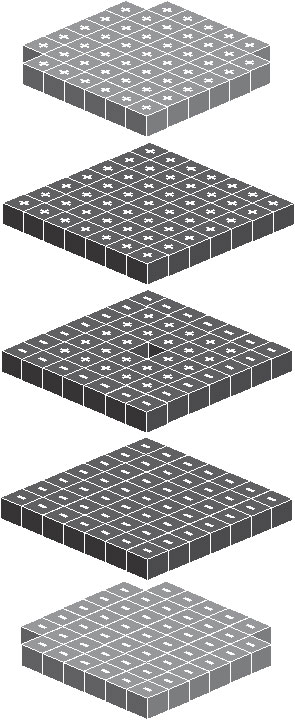
Inside the atom, the electron act like a unit cube and distribute on the surface of atom the same way as the proton distributes on the surface of nucleus, but with a much larger size. Below is the given structure.